Isotope Drawing
Isotope Drawing - Made with by wei zheng joe taylor. Identify the element and write symbols for the isotopes. Web isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, z) but a different number of neutrons, meaning that their mass number, a, varies. The atomic number of carbon is 6, which means. Web the number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number. While some students like manipulating solid objects, others prefer drawing. Science > ap®︎/college chemistry > atomic structure and properties > mass spectrometry of elements. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Web for example, tin ( z = 50) has 10 stable isotopes, but the elements on either side of tin in the periodic table, indium ( z = 49) and antimony ( z = 51), have only 2 stable isotopes each. Web an element with three stable isotopes has 82 protons. Web here, we describe the basics of metabolite measurement by ms, including sample preparation, metabolomic analysis, and data interpretation. This simulation is part of the phet project, a leading provider of free online stem resources. Science > ap®︎/college chemistry > atomic structure and properties > mass spectrometry of elements. Use the periodic table and bohr model to identify the name. Web draw a bohr model of the isotope on the paper ; Nuclei with magic numbers of both protons and neutrons are said to be “doubly magic” and are even more stable. I like to draw the analogy of taking a journey. Select your preferences below and click 'start' to give it a try! Your source for the latest research. Web the number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number. Web isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine. Replicate the examples above, but in this exercise, have the students draw the structure. The doe isotope program addresses this need. Ap®︎/college chemistry > unit 1. Web an element with three stable isotopes has 82 protons. Isotopes can be defined as the variants of chemical elements that possess the same number of protons and electrons, but a different number of. Web an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties. Isotopes can be defined as the variants of chemical elements that possess the same number of protons and electrons, but. Visualize trends, 3d orbitals, isotopes, and mix compounds. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate the average atomic mass (weight) of all atoms of an element taking into account the mass of each isotope present and the percent abundance for each isotope. However, isotopes are not always. These represent the first batch of new isotopes made at frib, a user facility for the u.s. While some students like manipulating solid objects, others prefer drawing. This quiz aligns with the following ngss standard (s): Web how to draw the atomic structure of atoms. I like to draw the analogy of taking a journey. Web isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, z) but a different number of neutrons, meaning that their mass number, a, varies. This simulation is part of the phet project, a leading provider of free online stem resources. Try this interactive simulation and explore the structure and symbols of atoms,. Web the number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number. Web draw a bohr model of the isotope on the paper ; Have students draw different isotopes of the same element with pens or markers. Use red ink for protons and. However, isotopes are not always available in sufficient quantities or at reasonable prices. Every chemical element has one or more isotopes. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Web here, we describe the basics of metabolite measurement by ms, including sample preparation, metabolomic analysis, and data interpretation. Web isotope notation,. However, isotopes are not always available in sufficient quantities or at reasonable prices. Your source for the latest research news. Additionally, n = a −z. Select your preferences below and click 'start' to give it a try! Web the number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number. Web draw a bohr model of the isotope on the paper ; Nuclei with magic numbers of both protons and neutrons are said to be “doubly magic” and are even more stable. Try this interactive simulation and explore the structure and symbols of atoms, isotopes, and ions. Web for example, tin ( z = 50) has 10 stable isotopes, but the elements on either side of tin in the periodic table, indium ( z = 49) and antimony ( z = 51), have only 2 stable isotopes each. Web do you want to learn how to build an atom from scratch? Web how to draw an isotope. Isotopes can be defined as the variants of chemical elements that possess the same number of protons and electrons, but a different number of neutrons. Use red ink for protons and black ink for electrons. The atomic number of carbon is 6, which means. Use the periodic table and bohr model to identify the name of the isotope This quiz aligns with the following ngss standard (s):
Isotopes — Definition & Overview Expii
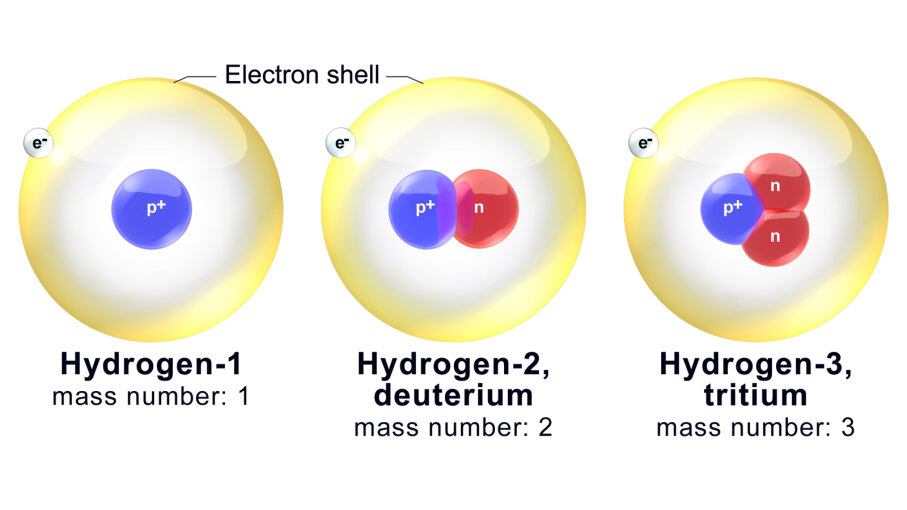
3. Isotopes
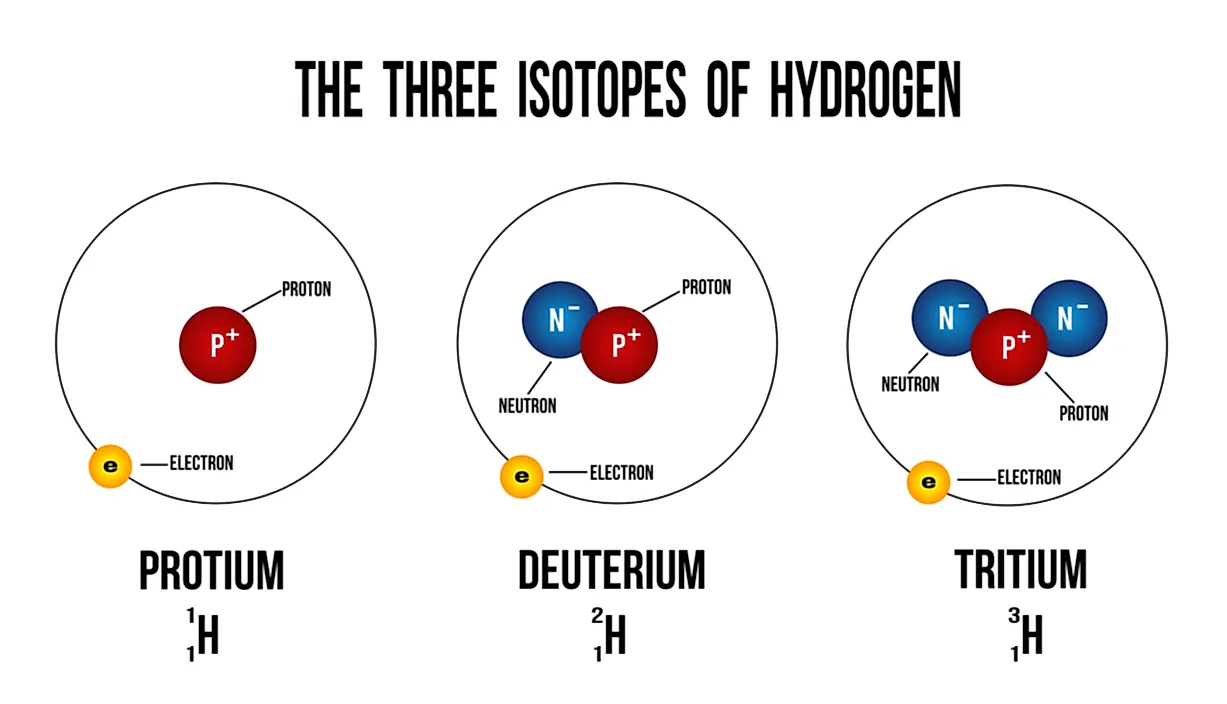
What Is an Isotope? WorldAtlas
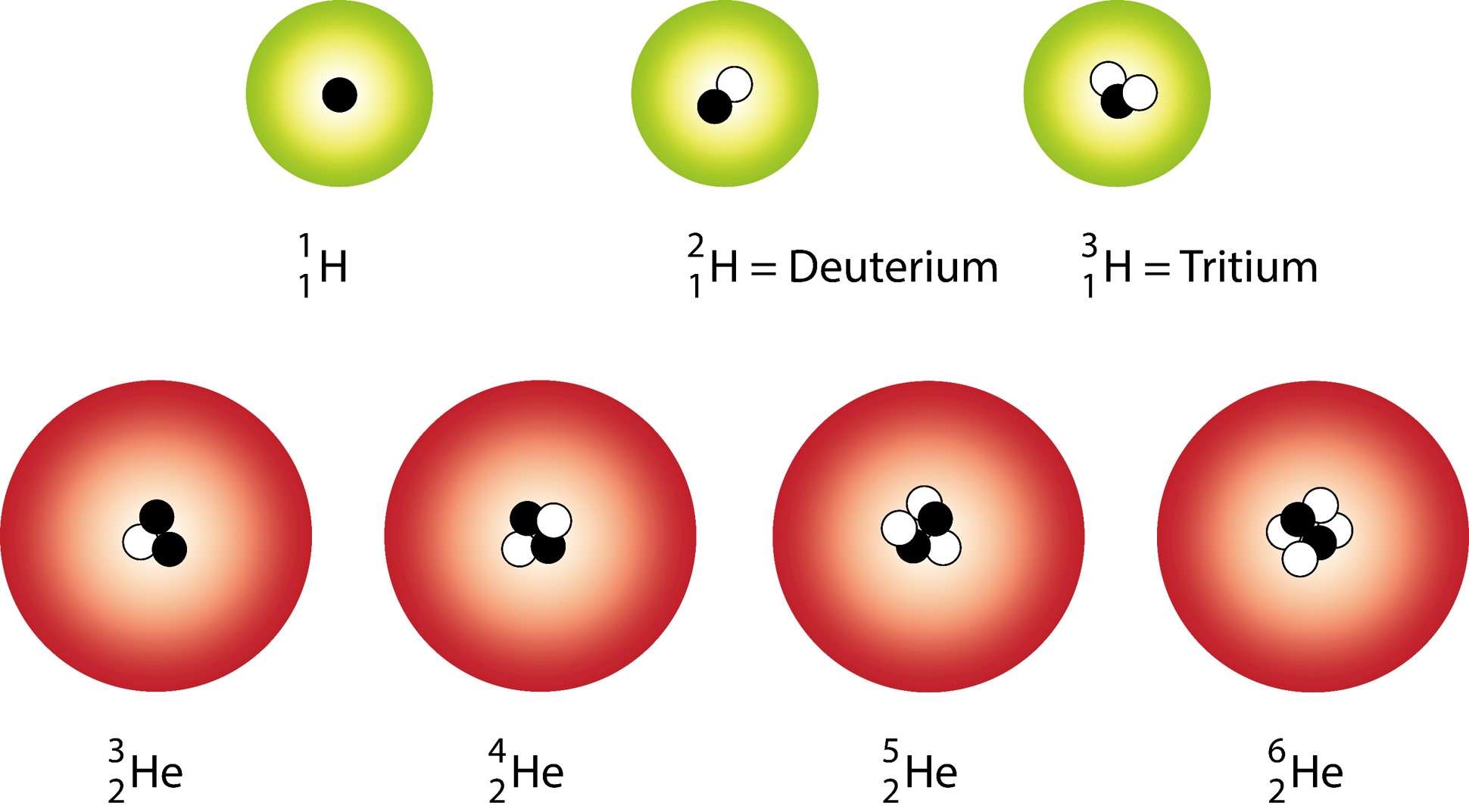
PreChemistry
How to Calculate Isotopes Sciencing
/Isotope-58dd6b415f9b5846830254ae.jpg)
Isotopes Definition and Examples in Chemistry
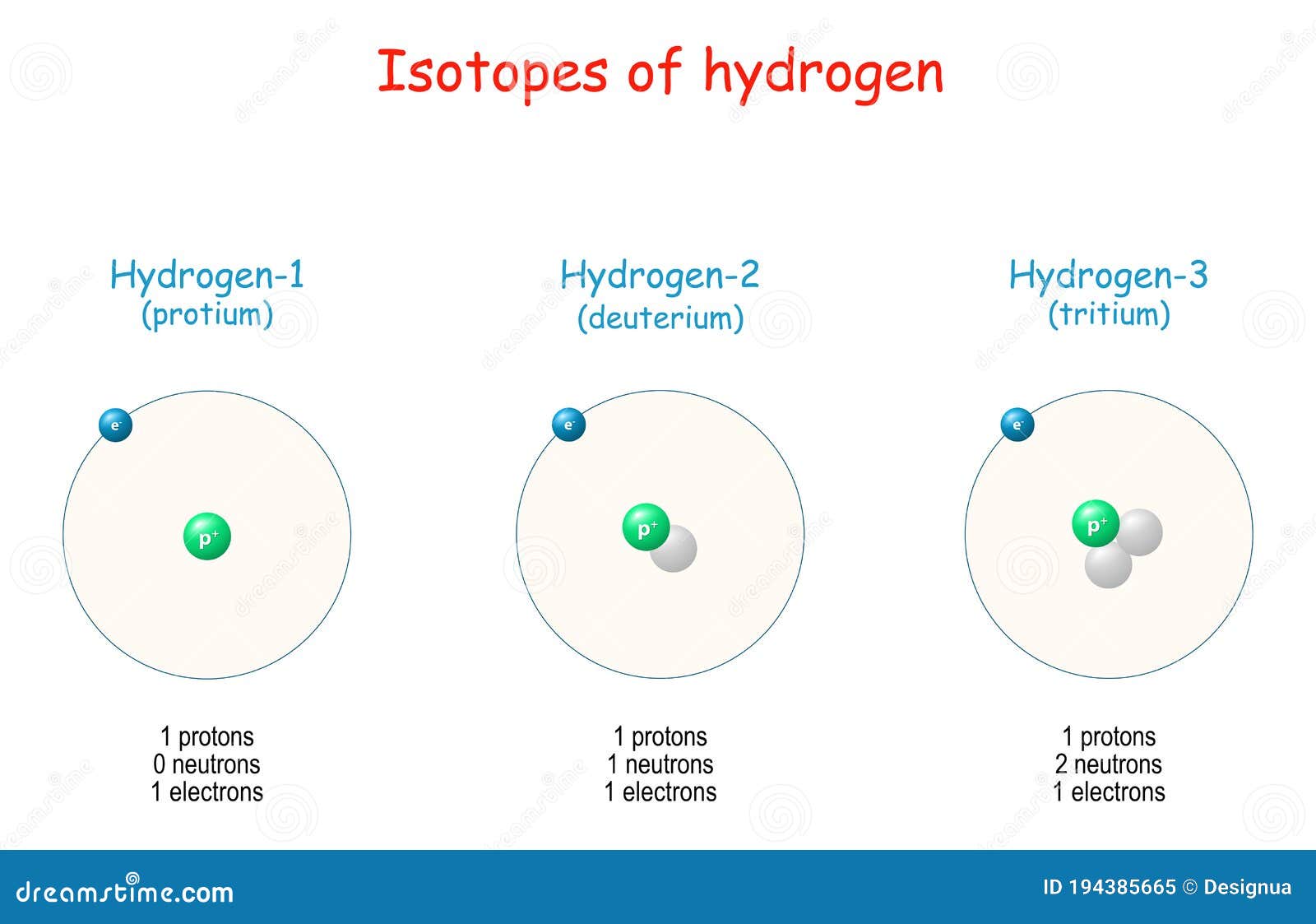
Hydrogen Isotopes. Atomic Structure Stock Vector Illustration of
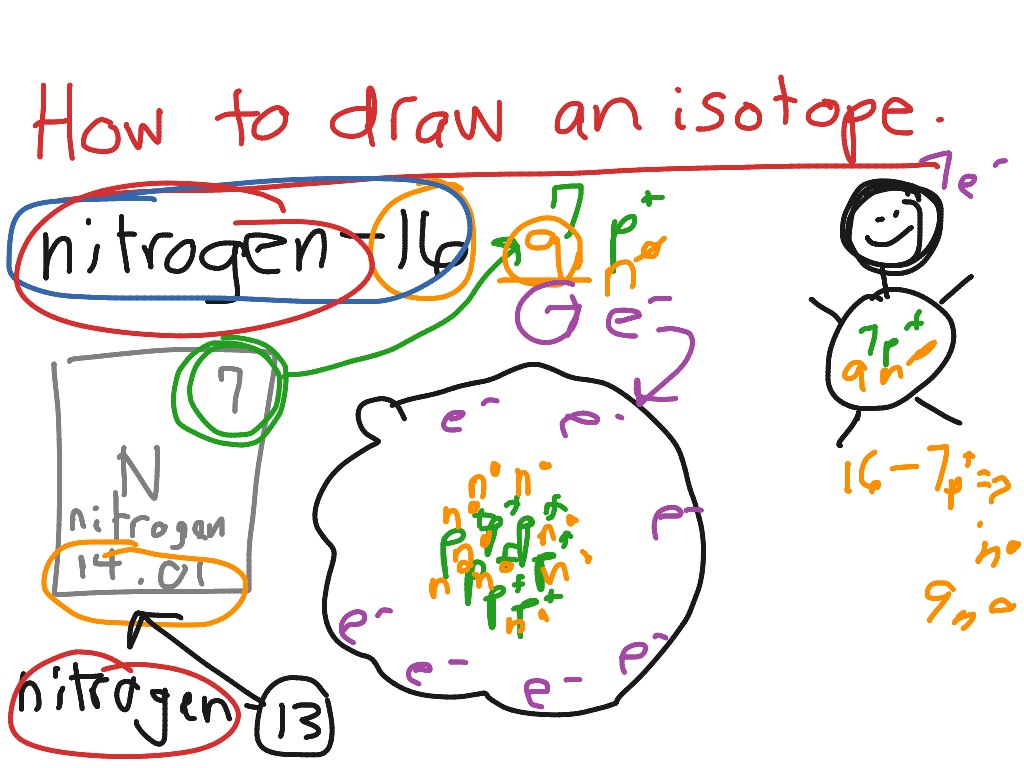
How to Draw an Isotope Science ShowMe

Isotopes of carbon, illustration Stock Image C028/6464 Science
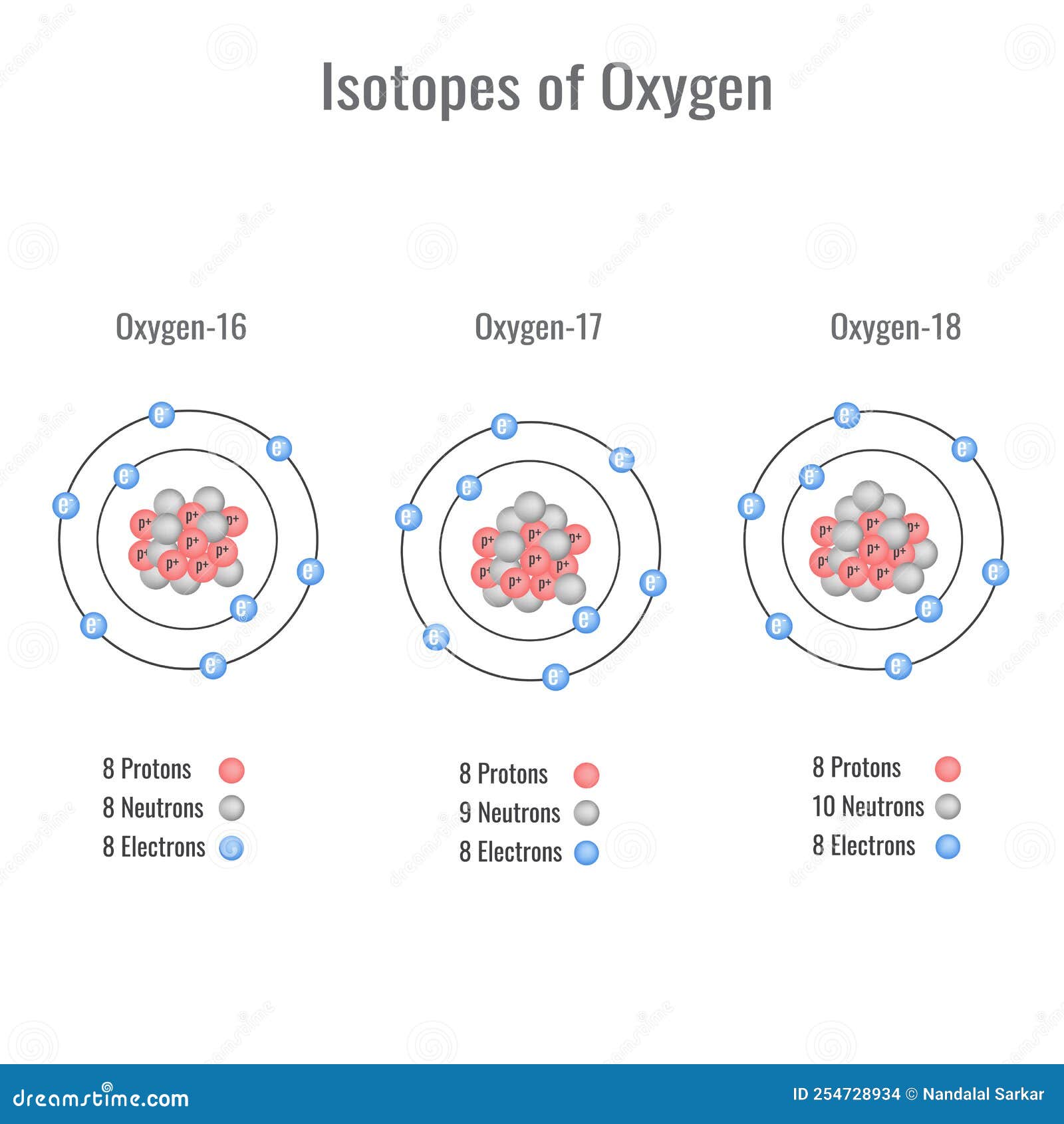
Isotopes of Oxygen Vector Illustration Stock Vector Illustration of
Web Isotopes Are Atoms Of The Same Element That Have The Same Number Of Protons (I.e., Atomic Number, Z) But A Different Number Of Neutrons, Meaning That Their Mass Number, A, Varies.
The Separate Isotopes Contain 124, 125, And 126 Neutrons.
Every Chemical Element Has One Or More Isotopes.
Number Of Protons And Neutrons.
Related Post:
