Draw The Main Lewis Structure Of Nof
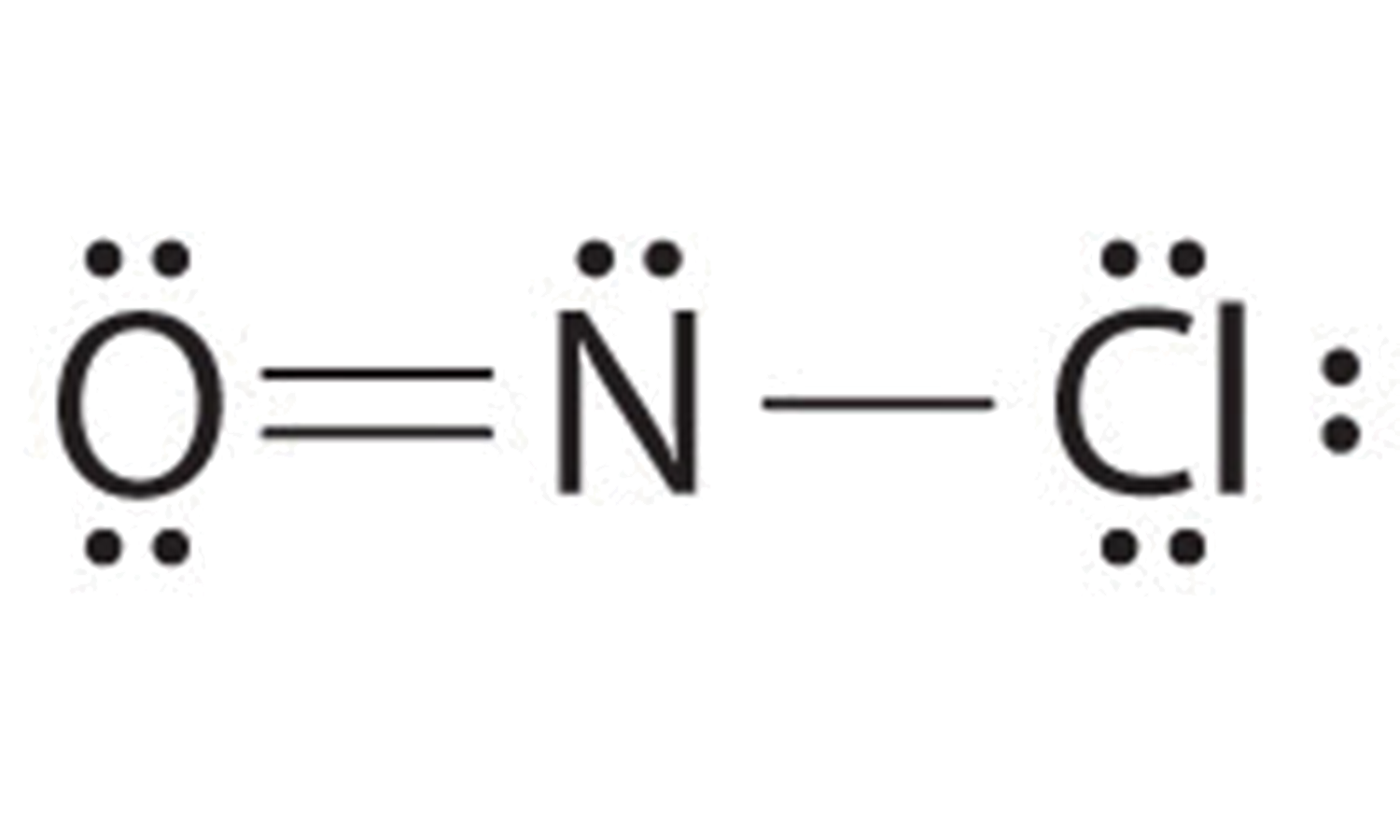
Draw The Main Lewis Structure Of Nof - Draw the lewis structure for nof. Here, the given molecule is nof. Draw the lewis structure for nof. First determine the total number of valence electrons in the molecule. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web the first step is to draw the lewis structure of the molecule. When drawing the structure of an ion, be sure to add/subtract electrons to account for. In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in the nof molecule. Part b draw the main lewis structure of nof. Find the total valence electrons in nof molecule. Draw the main lewis structure of nof. #4 complete octet on central atom. Web use these steps to correctly draw the nof lewis structure: In the nof lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. For \(\ce{pcl3}\), the electron dot diagram is as follows: Check the formal charges to be sure that each atom has a formal charge of zero. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. For the nof structure use the periodic table to find the total number. In the nof lewis structure nitrogen (n) is the least electronegative atom and goes in. Draw the main lewis structure of nof. Web 46k views 10 years ago. Here, the given molecule is nof. Web this video illustrates the thinking behind determining the lewis structure of a simple molecule and using that information to determine the electron pair and. Calculate the total number of valence electrons. #4 complete octet on central atom. In the nof lewis structure nitrogen (n) is the least electronegative atom and goes in the center of the lewis structure. #5 calculate formal charge and check stability. Web chemistry questions and answers. #3 calculate and mark formal charges on the atoms, if required. It was introduced in 1916 by gilbert n. #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. This problem has been solved! Web chemistry questions and answers. Find the total valence electrons in nof molecule. A) draw the lewis structure for no. #5 calculate formal charge and check stability. Here, the given molecule is nof. #3 calculate and mark formal charges on the atoms, if required. #4 complete octet on central atom. Find the total valence electrons in nof molecule. View available hint (s) ννου ch on spf brax more. A) draw the lewis structure for no. Determine the number of bonding electrons and the number of nonbonding electrons in. The lewis structure for nof is: Web the lewis structure of nof is drawn by first tallying up the total number of valence electrons from all the atoms, which in this case is 5 from nitrogen, 6 from oxygen, and 7 from fluoride (total 18 electrons). Web use these steps to correctly draw the nof lewis structure: This will be the sum of the group number. Draw nonbonding electrons using the dot notation and bonding electrons as a bond. #2 mark lone pairs on the atoms. Draw the main lewis structure of nof. Include all lone pairs of electrons. Web steps of drawing nof lewis structure step 1: Draw the main lewis structure of nof. Web the first step is to draw the lewis structure of the molecule. Web draw the lewis structure for nof nitrogen is the central atom draw the molecule by placing atoms on the grid and connecting them with bonds. #4 convert lone pairs of the atoms, and minimize formal charges. Nof is actually. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Include all lone pairs of electrons. This problem has been solved! Web 453 views 1 year ago lewis structure. Web the first step is to draw the lewis structure of the molecule. Web chemistry questions and answers. First determine the total number of valence electrons in the molecule. Draw the main lewis structure of nof. Let’s one by one discuss each step in detail. In order to draw the lewis structure of nof, first of all you have to find the total number of valence electrons present in the nof molecule. For the nof structure use the periodic table to find the total number. Web the nitrogen is double bonded to the oxygen atom on one side and it is single bonded to the fluorine on the other side. Calculate the total number of valence electrons. Web chemistry questions and answers. #1 first draw a rough sketch. Draw the lewis structure for nof.
Draw the main lewis structure of nof. draw nonbonding electrons using

NOF Lewis Structure, Geometry, Hybridization, and Polarity

How to draw lewis structures for NOF in 60s! Dr K shorts YouTube
Solved Part B Draw the main Lewis structure of NOF. Draw

NOF Lewis structure, molecular geometry, bond angles, resonance

NOF Lewis Structure How to Draw the Lewis Structure for NOF YouTube
Main Lewis Structure Of Nof

Draw The Main Lewis Structure Of Nof.
Nof Lewis Structure With Charges
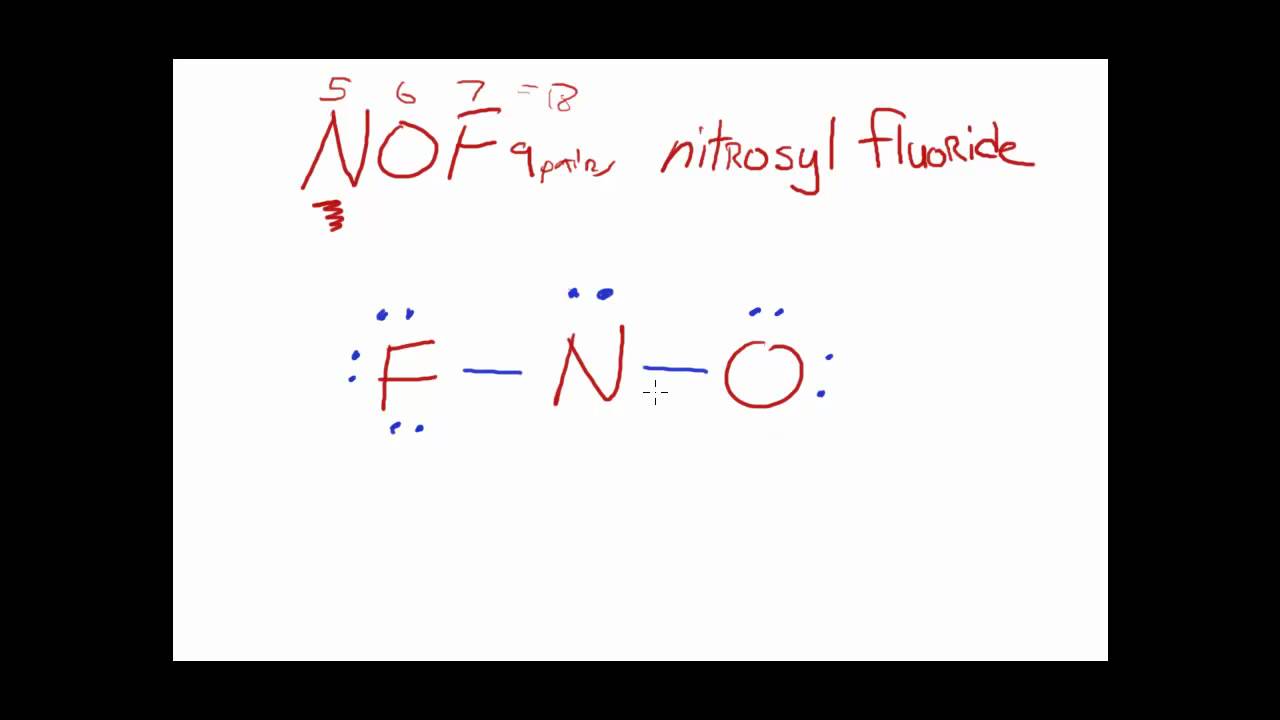
Structure and Geometry The NOF example YouTube
Draw The Lewis Structure For Nof.
This Problem Has Been Solved!
Web This Video Illustrates The Thinking Behind Determining The Lewis Structure Of A Simple Molecule And Using That Information To Determine The Electron Pair And.
Nitrogen Has The Five Valence Electrons.
Related Post: