Draw The Electron Configuration For A Neutral Atom Of Nickel
Draw The Electron Configuration For A Neutral Atom Of Nickel - Electron configuration of carbon (c) [he] 2s 2 2p 2: The neutral nickel atom therefore must have 28 electrons to accommodate according to the. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. The electron configuration follows the order: Web draw the electron configuration for a neutral atom of nickel. Using our example, iodine, again, we see on the periodic table that its atomic number is 53. Si 4 + was formed by the loss of four. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p,. 1s 2 2s 2 2p 6 3s 2 3p 2. Draw the electron configuration for a neutral atom of scandium. Draw the electron configuration for a neutral atom of scandium. 1s 2 2s 2 2p 6 3s 2 3p 2. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. 1s 2 2s 2 2p 1: Write the configuration of the neutral atom. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. We have chosen to show. A nickel nucleus has 28 positively charged nuclear particles, 28 protons. The electron configuration follows the order: Web electron configuration of boron (b) [he] 2s 2 2p 1: For example, the electron configuration of lithium, 1s²2s¹, tells us that. The electron configuration follows the order: 1s 2 2s 2 2p 2: Determine how many electrons were lost. Electron configuration of carbon (c) [he] 2s 2 2p 2: Si 4 + was formed by the loss of four. Web draw the electron configuration for a neutral atom of nickel. The atomic number of cl is 17. Electron configurations describe where electrons are located around the nucleus of an atom. The neutral nickel atom therefore must have 28 electrons to accommodate according to the. Determine how many electrons were lost. Electron configurations describe where electrons are located around the nucleus of an atom. 1s 2 2s 2 2p 1: Draw the electron configuration for a neutral atom of scandium. Web draw the electron configuration for a neutral atom of nickel. 1s 2 2s 2 2p 1: Web draw the electron configuration for a neutral atom of nickel. Using our example, iodine, again, we see on the periodic table that its atomic number is 53. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Nickel has an atomic number of. Electron configurations describe where electrons are located around the nucleus of an atom. The neutral nickel atom therefore must have 28 electrons to accommodate according to the. Web the neutral atom chlorine (z=17), for instance has 17 electrons. For example, the electron configuration of lithium, 1s²2s¹, tells us that. Using our example, iodine, again, we see on the periodic table. Determine how many electrons were lost. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. Web a nickel nucleus has 28 positively charged nuclear particles, 28 protons. 1s 2 2s 2 2p 6 3s 2 3p 2. Electron configuration of carbon (c) [he] 2s 2 2p 2: A neutral chlorine atom has 17. Draw the electron configuration for a neutral atom of scandium. We have chosen to show. The electron configuration follows the order: For example, the electron configuration of lithium, 1s²2s¹, tells us that. Web what is the electron configuration of: The neutral nickel atom therefore must have 28 electrons to accommodate according to the. We will now construct the. Draw the electron configuration for a neutral atom of iron. The atomic number of cl is 17. A nickel nucleus has 28 positively charged nuclear particles, 28 protons. Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration of carbon (c) [he] 2s 2 2p 2: We will now construct the. Nickel has an atomic number of 28, which means it has 28 electrons in a neutral atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that. Using our example, iodine, again, we see on the periodic table that its atomic number is 53. 1s 2 2s 2 2p 2: Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. 1s 2 2s 2 2p 6 3s 2 3p 2. Web electron configuration of boron (b) [he] 2s 2 2p 1: We have chosen to show. Draw the electron configuration for a neutral atom of scandium. The atomic number of cl is 17. Web the neutral atom chlorine (z=17), for instance has 17 electrons. For nickel, z = 28.
OneClass draw the electron configuration for a neutral atom of nickel.

Nickel Atom Science Notes and Projects
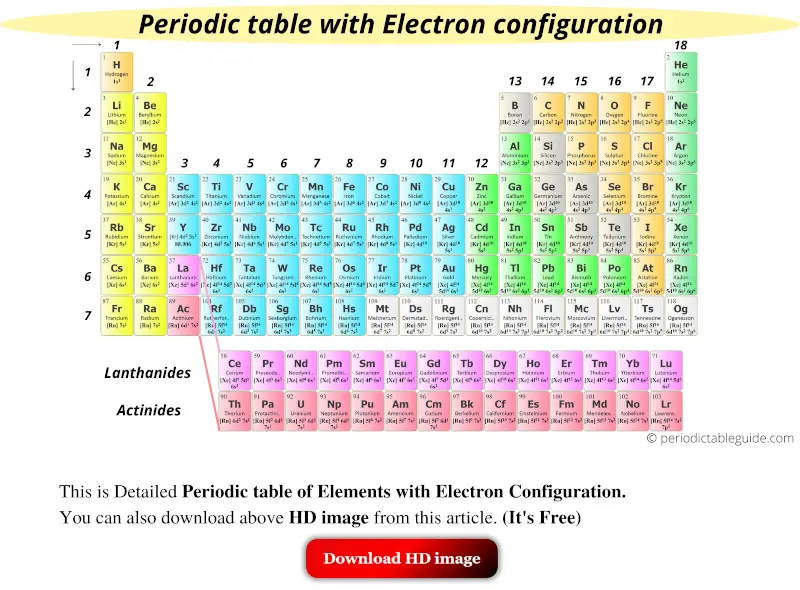
Get the Detailed Periodic table (With Electron Configuration)
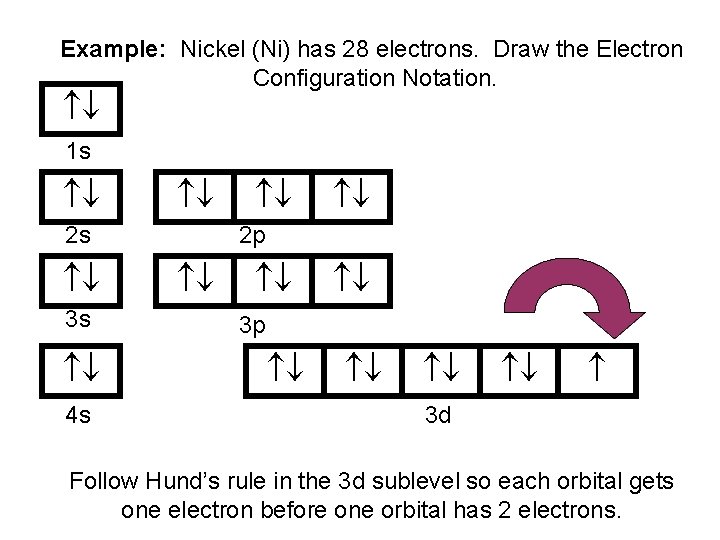
Electron Configuration Chapter 5 Electrons have 3 levels
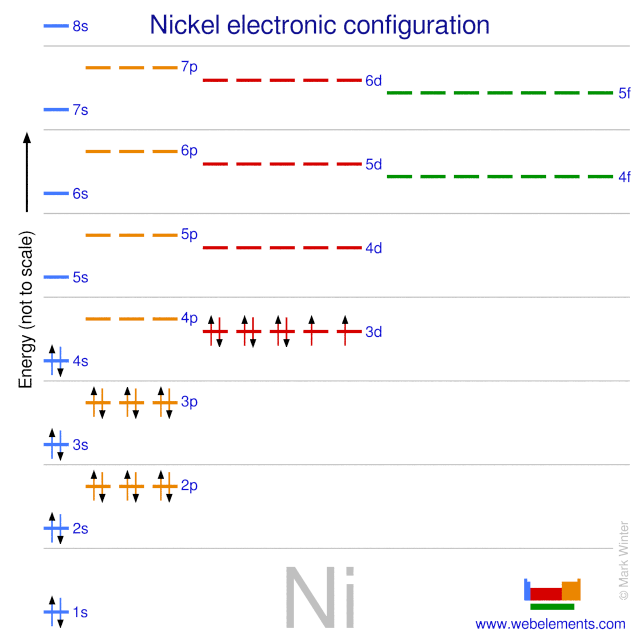
WebElements Periodic Table » Nickel » properties of free atoms
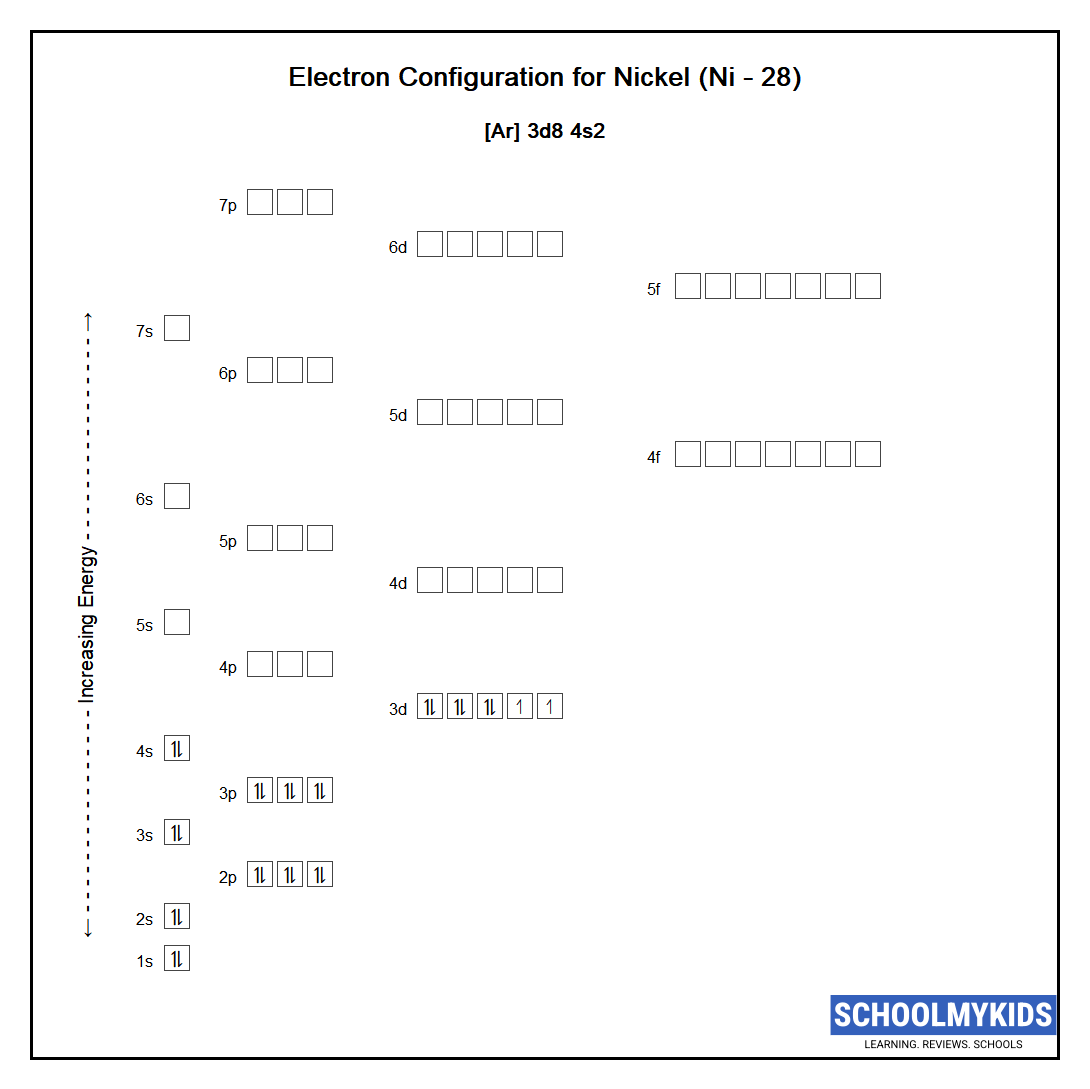
Nickel (Ni) Element Information, Facts, Properties, Uses Periodic

Symbol and electron diagram for nickel Royalty Free Vector

Nickel Electron Configuration 9 Facts You Should Know!

electron configuration of nickel YouTube
Electron Configuration For Nickel cloudshareinfo
Draw The Electron Configuration For A Neutral Atom Of Iron.
Web A Nickel Nucleus Has 28 Positively Charged Nuclear Particles, 28 Protons.
Write The Configuration Of The Neutral Atom.
Determine How Many Electrons Were Lost.
Related Post: