Draw All Resonance Structures For The Ozone Molecule O3
Draw All Resonance Structures For The Ozone Molecule O3 - Web draw the lewis structure for the ozone (o3) molecule be sure to include all resonance structures that satisfy the octet rule. Web share 31k views 3 years ago lewis structures ozone molecules are three oxygen atoms bonded in succession, they are not in a ring. Note, the two headed arrow means these are not real structures but resonance structures. If there are equivalent resonance structures, draw all of them. But as we draw lewis structures and we follow those. Draw lewis structure(s) for the ozone molecule (o3). It also discusses the molecular geometry, bond angle, hybridization, and formal charges. Web verified by toppr (i) resonating structure of ozone ( o 3 ): The total number of valence. Draw the lewis structure for the ozone (03) molecule. It also discusses the molecular geometry, bond angle, hybridization, and formal charges. 109k views 7 years ago. It is the diagrammatic layout for understanding the nitty. In ozone, a molecular orbital extending over all three oxygen. Web figure 5.3.4 the resonance structure of ozone involves a molecular orbital extending all three oxygen atoms. In nature, the actual ozone molecule, the o3 molecule, is not either one of these. Web share 31k views 3 years ago lewis structures ozone molecules are three oxygen atoms bonded in succession, they are not in a ring. Draw the lewis structure for the ozone (03) molecule. The total number of valence. Web a molecule or ion with such. In ozone, a molecular orbital extending over all three oxygen. That is, from figure 8.3.1 there. Web this chemistry video tutorial explains how to draw the lewis structure of o3. Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule. Ozone (o3) is an highly reactive gas that consists of. Draw all resonance structures for the ozone molecule, o3. The middle oxygen, according to the lewis. In nature, the actual ozone molecule, the o3 molecule, is not either one of these. Web verified by toppr (i) resonating structure of ozone ( o 3 ): Draw the lewis structure for the ozone (03) molecule. Web for example, drawing one lewis structure for ozone (o 3) gives us a misleading picture of the actual bonding in the molecule. Draw the lewis structure for the ozone (03) molecule. Web draw the lewis structure for the ozone (o3) molecule be sure to include all resonance structures that satisfy the octet rule. Web this chemistry video tutorial explains. In ozone, a molecular orbital extending over all three oxygen. The two lewis dot structures of ozone. If we draw a lewis structure. Web draw the lewis structure for the ozone (o3) molecule be sure to include all resonance structures that satisfy the octet rule. Draw lewis structure(s) for the ozone molecule (o3). Web to be very precise, lewis structure is the name given to the structural representation of a molecule. If we draw a lewis structure. Draw lewis structure(s) for the ozone molecule (o3). It also discusses the molecular geometry, bond angle, hybridization, and formal charges. Note, the two headed arrow means these are not real structures but resonance structures. After drawing the lewis structure of nh 3, the shape of the o 3 molecule can be. In nature, the actual ozone molecule, the o3 molecule, is not either one of these. The total number of valence. If we draw a lewis structure. Web there are two resonance structures for o3 (ozone). It also discusses the molecular geometry, bond angle, hybridization, and formal charges. Web draw the lewis structure for the ozone (o3) molecule be sure to include all resonance structures that satisfy the octet rule. That is, from figure 8.3.1 there. 109k views 7 years ago. Web verified by toppr (i) resonating structure of ozone ( o 3 ): Solved by verified expert video by. Web a molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). 18 similar questions q 1. In ozone, a molecular orbital extending over all three oxygen. If we draw a lewis structure. (figure 1) (ii) resonating structure of n o 3 −: If we draw a lewis structure. The lewis structure of ozone has: In ozone, a molecular orbital extending over all three oxygen. Be sure to include all resonance structures that satisfy the octet rule. If there are equivalent resonance structures, draw all of them. Ozone (o3) is an highly reactive gas that consists of three oxygen atoms. But as we draw lewis structures and we follow those. Draw lewis structure(s) for the ozone molecule (o3). Such is the case for. Solved by verified expert video by. Web there are two resonance structures for o3 (ozone). * three oxygen atoms in a row. Web this lesson walks you through each step of drawing the lewis structure of o 3. It is the diagrammatic layout for understanding the nitty. Web ozone, or #o_3#, has two major resonance structures that contribute equally to the overall hybrid structure of the molecule.
O3 Resonance Structures (Ozone) YouTube

Resonance Structures of O3, Ozone YouTube
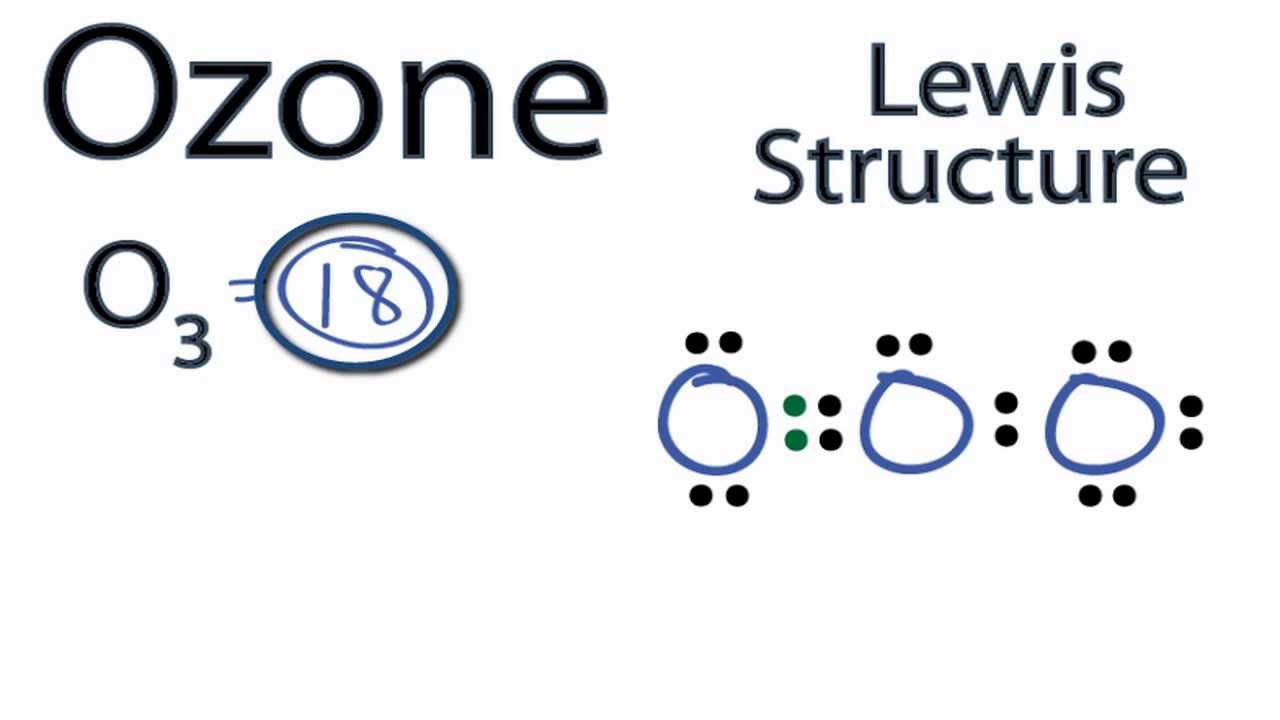
Ozone Lewis Structure How to Draw the Lewis Structure for Ozone YouTube
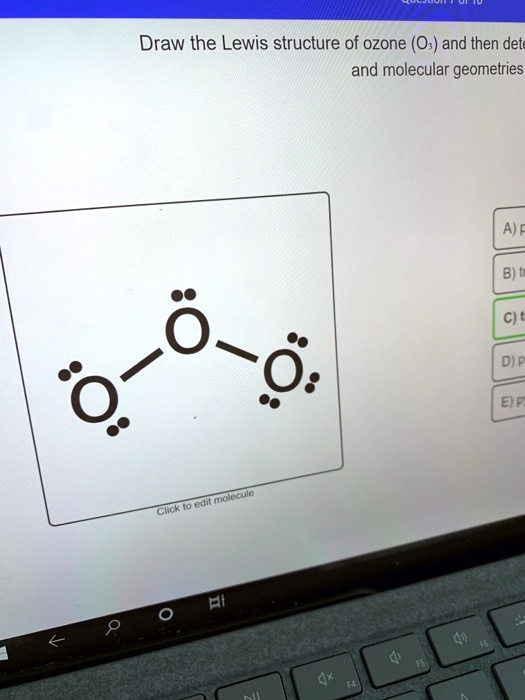
SOLVED Draw the Lewis structure of ozone (O3) and then determine its
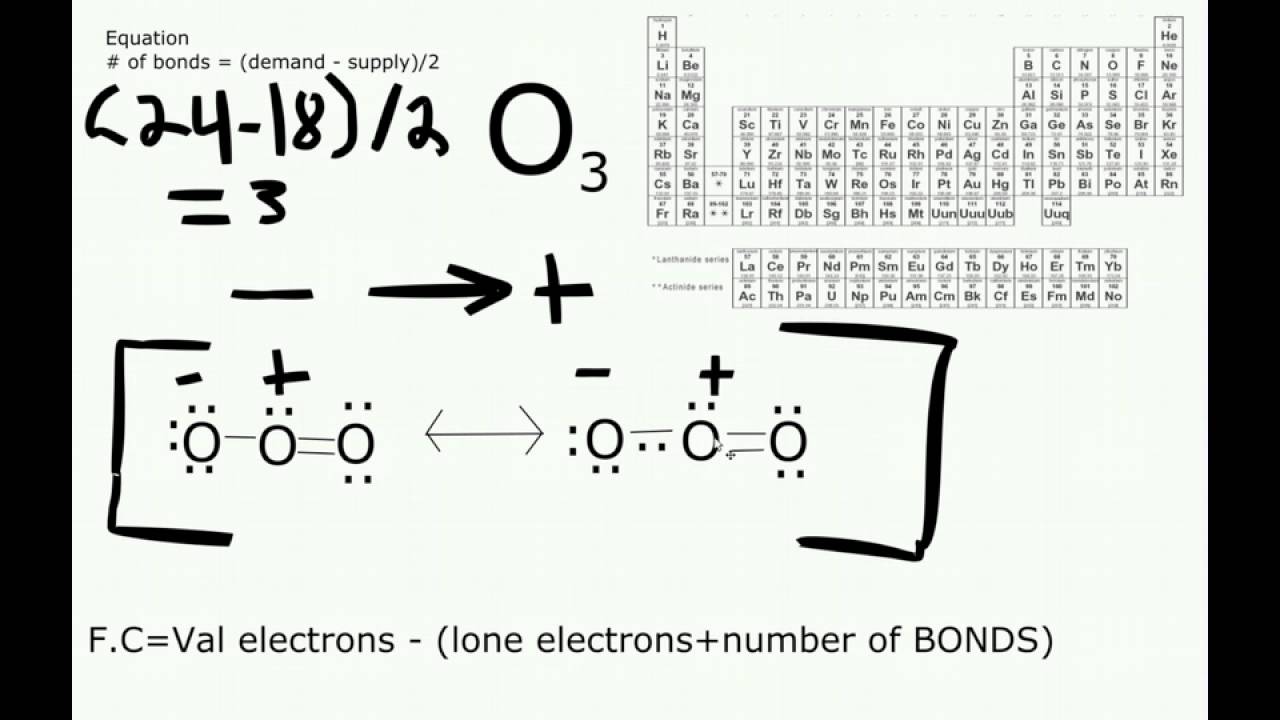
O3 Lewis Structure Ozone YouTube

Resonance Structures Easy Hard Science
Resonance Presentation Chemistry

O3 Lewis Structure Ozone YouTube
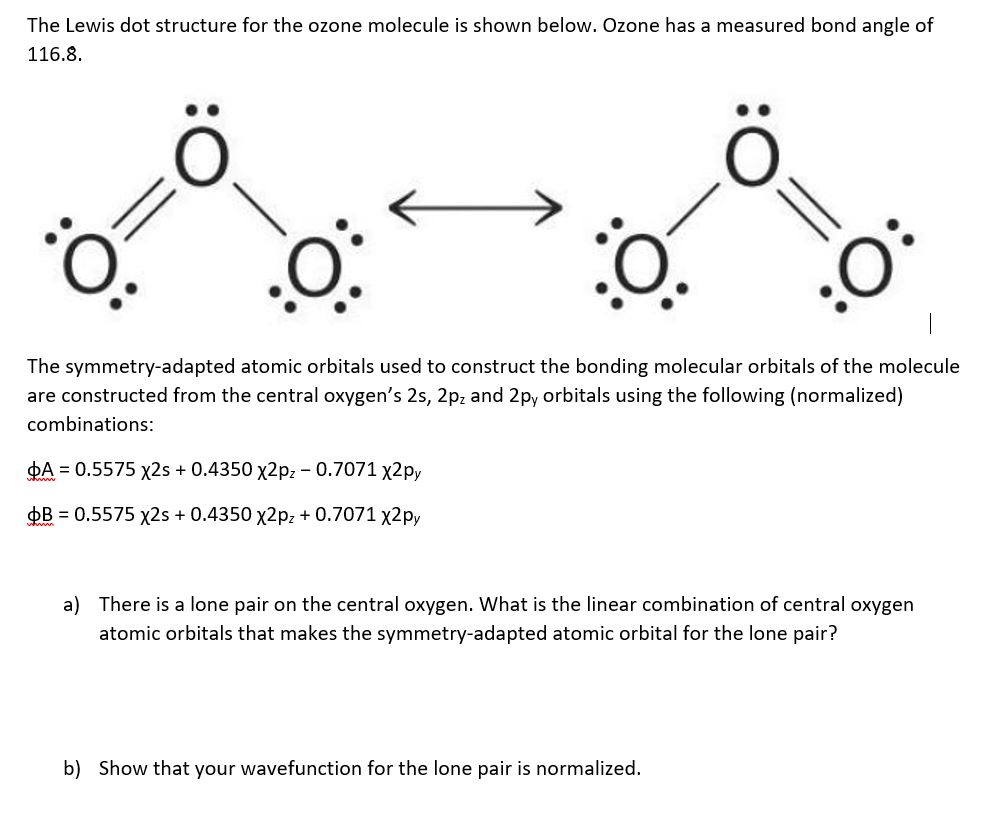
The Lewis dot structure for the ozone molecule is

Ozone Molecule Lewis Structure
Each Step Of Drawing The Lewis Structure Of O 3 Is Explained In Detail In.
Web To Be Very Precise, Lewis Structure Is The Name Given To The Structural Representation Of A Molecule.
For The O3 Structure Use The Periodic Table To Find The Total Number Of Valence.
Web Figure 5.3.4 The Resonance Structure Of Ozone Involves A Molecular Orbital Extending All Three Oxygen Atoms.
Related Post:
.PNG)